Fusion - Energy Production in Stars
Overview
The purpose of this page is to describe the fusion energy-making processes inside stars in one location, rather than have the information duplicated and/or spread about on multiple pages in multiple sections of the site.
The reason that this can be done is that all "live" stars produce their energy through some form of fusion, which is making energy by combining smaller atoms to make bigger atoms.
The way this releases energy is that atoms that are heavier than others (until you get to iron, Fe) weigh slightly less than the atoms that were combined to make it. The extra mass has to go somewhere, and from Einstein's famous equation ![]() , that extra mass can be (and is) converted and released as energy.
, that extra mass can be (and is) converted and released as energy.
There are many different types of fusion processes, but the main ones during normal stellar life is some variation of turning hydrogen into helium. In Sun-like stars, this is generally done through a combination of the different PP chains, so-named because they all start with combining two protons. In more massive stars, the CNO cycle takes over as the main energy producer. The reason that some processes dominate over others in different stars is that the probability of the collision happening is dependent upon the temperature.
After a star has generally exhausted its supply of hydrogen fuel, if it is massive enough it will be able to fuse heavier elements together, such as helium, silicon, and oxygen. The more massive a star, the higher the core's temperature and pressure can get, and so the more massive elements it can fuse together.
A star cannot fuse elements past iron. This is because, up until iron, fusion will release energy. But fusing elements heavier than iron requires energy to be input, which causes the star to collapse due to its own weight.
This page will not address when stars switch to different fusion products, stages of stellar evolution (Sun-like, Giants), what exactly the sun does, nor will it discuss specifics of particle physics such as what a neutrino or positron is. For that information, please see those specific sections of the site (for example, as linked in this paragraph).
The PPI Chain - Simple Hydrogen Fusion
Stars like the sun primarily produce energy through the PPI process, which is pronounced "P-P One" because it is the main proton-proton fusion reaction. This is a three-step process even though it is the second step that produces the light that we see:
- Two protons (hydrogen atoms that have been stripped of their neutrons) combine to produce a hydrogen atom with one proton and one neutron, known as deuterium. Because neutrons weigh slightly less than a proton, two by-products that are released are a neutrino and positron (the anti-matter counterpart of an electron). The positron will combine with an electron and release some energy.
- A third proton combines with the hydrogen atom to produce a form of helium known as helium-3, which is two protons and one neutron. The by-product of this fusion step is a photon, which is the energy that we generally think of.
- Two helium-3 nuclei combine to form a helium-4 nucleus. Because what was combined were 4 protons and 2 neutrons, but helium-4 only has 2 protons and 2 neutrons, 2 protons are released as by-products from this step, free to fuse again. Most of the energy of this process is release in this step.
In chemistry notation, this chain is written as:
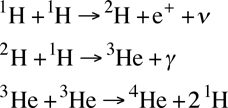
The net result of this is a release of 26.7 MeV of energy, where 1 eV is 1.6022·10-19 Joules. This may seem like a very tiny amount of energy, but keep in mind that about 2·1045 of these reactions take place every second in our sun. This is often referred to as "hydrogen burning."
The "choice" of which proton-proton chain is based on temperature-dependent probabilities. Between about 10-14 million Kelvins, the PPI chain is dominant.
The PPII Chain - Lithium and Beryllium as Intermediates
A rarer form of fusion in Sun-like stars is the PPII chain, which fuses elements through lithium and beryllium but destroys them in the end, effectively using them as catalysts:
- This process starts with an atom of helium-3 (from the second step of the PPI chain) and helium-4 (the end product of the PPI chain) and fusing them into beryllium-7. This is an atom with 4 protons and 4 neutrons. It releases a photon in the process.
- The next step is to take the beryllium-7 and to combine it with an electron (known as electron capture) which allows it to decay into lithium-7 which has 3 protons and 4 neutrons. This releases both a neutrino and energy. This is a reasonably fast step, with a half-life calculated at about 150 days for a star like the sun.
- The final step takes the lithium-7 and combines it with deuterium (from the first step of the PPI chain) to create two atoms of helium-4. This is a very fast step with a half-life calculated at about 10 minutes for a star like the sun.
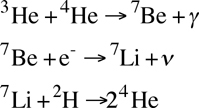
The PPII chain is also sometimes referred to as "lithium burning." It will become the dominant of the three main proton-proton processes in a star with a core temperature between 14-23 million Kelvins.
The PPIII Chain - Lithium, Beryllium, and Boron as Intermediates
An even rarer form of fusion in Sun-like stars is the PPIII chain, which fuses elements through lithium, beryllium, and boron, but destroys them in the end, effectively using them as catalysts:
- This process starts with an atom of beryllium-7 (from the first step of the PPII chain) and a proton (known as proton capture), and it fuses them into boron-8. This is an atom with 4 protons and 4 neutrons. It releases a photon in the process. In the sun, this is a process that takes ~200 times as long as Step 2 of the PPII chain, which is why it is so rare for it to happen in our star.
- The next step is the boron-8, which is highly unstable, will rapidly decay into beryllium-8 plus a positron and neutrino (plus energy).
- Berilium-8 is also highly unstable, and it will quickly decay into 2 helium-4 nuclei, releasing energy in the process.
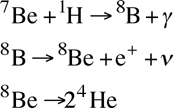
The PPIII chain will become the dominant of the three main proton-proton processes in a star with a core temperature above 23 million Kelvins.
The CNO Cycle
For the very first stars, only the PP chains could create energy because the stars lacked any heavier elements. However, second and third generation stars (like our own sun) have enough heavier elements that they can be used in the fusion process.
In the case of the CNO cycle, carbon, nitrogen, and oxygen are used as catalysts for hydrogen-to-helium fusion. The CNO cycle is not efficient at low temperatures like those found in Sun-like stars. It is estimated that only 1.7% of the helium-4 nuclei produced in our star (with a core temperature of 15.7 million Kelvins) come from the CNO cycle.
However, at higher temperatures, such as above 17 million Kelvins, the CNO cycle starts to become the dominant energy source. The temperature-dependence is very large for this cycle, ![]() . This is contrasted with the PP chains, where energy production generally goes as
. This is contrasted with the PP chains, where energy production generally goes as ![]() .
.
The CNO cycle is best-illustrated in the figure below:
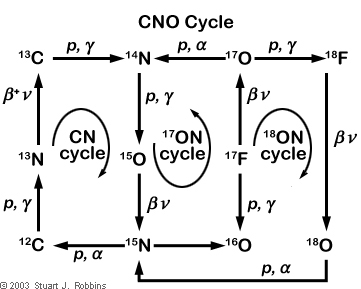
This diagram shows three different cycles that feed into each other, separated by the two catalysts that are used - carbon and nitrogen, oxygen-17 and nitrogen, or oxygen-18 and nitrogen. In this diagram, p is used to indicate a proton while α indicates a helium nucleus (also known as an "alpha particle"). For the other symbols, consider reading the Overview of Particle Physics sub-section of this website.
Helium Fusion - The Triple-α Process
Helium fusion generally occurs only once temperatures rise to nearly 10 times the core temperature of the sun - about 100,000,000 Kelvins. The reason that it requires such high temperatures is that, as implied by the name of this fusion process, three helium nuclei need to be close enough to interact and fuse together. At lower temperatures, the probability of this happening is very small:
- Two helium-4 nuclei fuse to form beryllium-8.
- Beryllium-8 has a very short half-life, as mentioned in Step 2 of the PPIII chain. If a third helium-4 nucleus doesn't fuse before the beryllium decays, then we will simply have the PPIII chain. If the extra helium-4 nucleus does fuse, however, then the product will be a carbon-12 nucleus plus a photon and energy.
The net energy release from this process is 7.367 MeV, which is only about 25% of the PPI chain. However, the likelihood of this process occurring and the speed with which it happens will be significantly greater in very hot stellar cores.
Note that, by the capture of an additional helium-4 nucleus, oxygen-16 can be created, and if a fifth helium-4 nucleus is fused, then neon-20 will be created.
Advanced Nuclear Fusion Processes
As stars use the last of their helium fuel, if they are massive enough then they will collapse far enough and temperatures will grow large enough that other elements can be fused together.
The different reactions shown below do not show each individual step to go from the start to the end, but rather they just illustrate possible end-states of each fusion process.
Carbon Fusion: Carbon fusion is the next step, and it generally requires temperatures in excess of 600-700 million Kelvins.
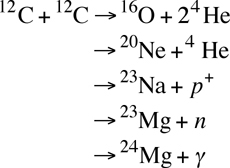
Each line is a possible end-product of the fusion of two normal carbon nuclei (6 neutrons and 6 protons):
- The first, oxygen and helium products, are endothermic, meaning that it actually requires energy to occur (0.114 MeV).
- The second line creates neon and helium, and it releases 4.616 MeV of energy.
- The third possible outcome is sodium plus a proton, and it releases 2.238 MeV of energy.
- Fourth is magnesium-23 and a neutron, which is endothermic like the first reaction, requiring 2.605 MeV to occur.
- A fifth possible outcome is magnesium-24 plus a photon, and this releases the greatest amount of energy of the carbon fusion, 13.930 MeV.
Neon Fusion: Neon fusion occurs between carbon and oxygen fusion. There are several different forms of it, in general fusing a neon atom with a helium nucleus to produce magnesium-24.
Oxygen Fusion: Oxygen fusion is the next step past carbon fusion. It requires temperatures of greater than 1 billion Kelvins.
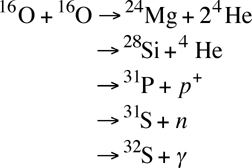
As with the carbon fusion above, each line is a possible end-product of the fusion of two normal oxygen nuclei (8 neutrons and 8 protons):
- The first, producing magnesium and two helium atoms, is endothermic, requiring 0.393 MeV of energy to occur.
- The second reaction produces silicon and helium, and it releases 9.593 MeV of energy.
- Third produces phosphorus and a helium nucleus, and it releases 7.676 MeV of energy.
- The fourth possible fusion process creates sulfur-31 plus a neutron, and it releases 1.459 MeV.
- A fifth possible end-product is sulfur-32 plus a photon, and this releases the most energy of the different oxygen fusion processes, 16.539 MeV.
Silicon Fusion: Silicon fusion in stars is the last stage of fusion. It is an all-encompassing term where elements between silicon (14 protons) and iron (26 protons) are created by alternating processes of fusion and radioactive decay.
The fusion builds the atoms up, and involves adding helium nuclei to silicon and then heavier elements (hence increasing the atomic mass number by 4 (2 protons and 2 neutrons)). If helium cannot be added fast enough before the atom decays, then it will generally lose protons and/or neutrons, which will produce the "intermediate" elements (such as potassium, which has 19 protons).
Silicon fusion requires temperatures in excess of 2.5 billion Kelvins.
Lifetimes of Heavier Fusion Processes: As a rule of thumb, each higher fusion process creates less and less energy than the previous cases. In addition, there is much less of each successively heavier element available for the star to fuse, and less of a volume within the star that can reach temperatures and pressures large enough for the fusion to take place. Because of this, stars can't spend nearly as much time "surviving" on these fusion processes. For example, the following table lists timescales for how long a star 15 times the mass of the sun can undergo each fusion process:
Reaction |
Timescale |
Hydrogen |
10,000,000 years |
Helium |
1,000,000 years |
Carbon |
300 years |
Oxygen |
200 days |
Silicon |
2 days |