Light (The Electromagnetic Spectrum)
Overview
The purpose of this page is to provide a fundamental overview of the electromagnetic spectrum ... otherwise known as "light." Studying different types of light is incredibly important in astronomy because it is one of the three main things that we can observe (the other two being gravity and magnetic fields).
The first section of this page will very briefly go over the nature of light and how it can be thought of in terms of color, energy, or frequency, as well as very briefly touching on the wave-particle duality of light.
The bulk of this page is dedicated to talking about different energy levels of light, running from the most energetic - gamma rays - to the least energetic - radio waves - and what each can be used for in astronomy. Note that these names of light (such as x-rays or microwaves) is a purely human convention, and in general, people disagree as to where exactly one "type" of light stops and another starts. The only difference between the different types of light is its inherent energy.
The final section discusses the concept of a black-body spectrum, which is how an object that is a "perfect emitter" emits light of different wavelengths, and how the energy level of the light is solely based upon the temperature of the object. This is important for understanding why some stars are blue while others are red, as well as understanding why we may have evolved to see the wavelengths of light that we can see.
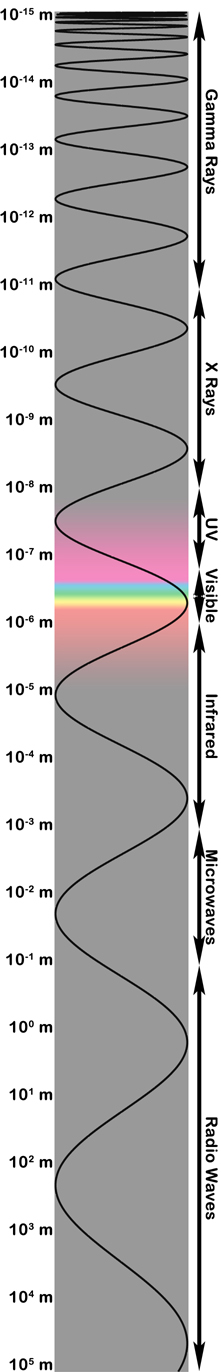 |
| The electromagnetic spectrum showing wavelength on the left and the approximate range of "types" of light on the right. Figure created by Stuart Robbins. |
Nature of Light (Briefly)
Despite it being one of the most important information-carrying components in our existence, we actually do not have a very good idea of what light actually is. For starters, is light a particle or a wave?
The Wave-Particle Duality: Unfortunately, light is neither a wave nor a particle, but it displays properties of both. For example, Albert Einstein won a Nobel Prize not for his formulation of Relativity, but for the discovery of the Photoelectric Effect. This is an experiment which showed that light acts like a particle.
However, there are other cases where light behaves like a wave, such as waves on the ocean or sound waves that let you hear. The experiment which shows this is the famous Double-Slit Experiment, which is where light is shone through two very small stretched-out holes (slits).
If light were a particle, then after it passed through the slits you would expect to see just two vertical spots of light (each particle of light choosing one slit or the other to go through). However, what you actually see is many slits repeated over and over again, to either side of the two main slits of light. This shows that the light has interfered with itself, making both bright spots and dark spots, which only a wave can do.
Color, Energy, Frequency, or Wavelength: Besides getting a headache thinking of light as both a wave and a particle (or as neither a wave nor a particle), there is a more practical way to think of light that most people do every day: Color.
Color, however, is a very imprecise way of thinking about light. I could say that my notebook is "red," but paint companies have invented several hundred different shades of red. Besides this, "color" can only refer to the kinds of light that we can see, the very narrow Visible Light.
Because of this, there are three other ways to think about light: Energy, Frequency, and Wavelength. Fortunately, these are all completely interchangable by this relationship:
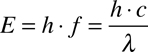
In the above equation, E is energy, h is Planck's Constant, f is frequency (measured in Hz), c is the speed of light, and λ is wavelength (measured in length; in astronomy, usually in Å (Ångströms)). This shows that, by using Planck's constant and the speed of light, you can refer to light as having a specific energy, a frequency, or a wavelength.
In high-energy astronomy, we generally refer to light as having a specific energy. In UV, optical, or IR astronomy, we generally refer to light as having a certain wavelength. In low-energy astronomy, we generally refer to light as having a specific frequency. I honestly don't know why these different observational branches have different nomenclature for the same thing, but nonetheless, they do.
Being more of a visible-light person, I have used wavelength to illustrate the electromagnetic spectrum in the image on the right.
High-Energy Light: Gamma and X Rays
a
Ultra-Violet Light
a
Visible Light
Red ~ 750-620 nm |
Orange ~ 620-590 nm |
Yellow ~ 590-570 nm |
Green ~ 570-495 nm |
Blue ~ 495-450 nm |
Violet ~ 450-380 nm |
The visible light range is defined to be the range of light that humans can see. In general, this is from about 380 nm to 750 nm. However, this varies from person-to-person, some people being able to see a little bit less and some a little more. The colors that we preceive that correspond to the various wavelengths are shown in the table on the right. Note that the ranges are approximate, and everyone will perceive minor variations.
The vast majority of astronomical images are taken in visible light, and this is mainly for two reasons. First, it is what we have used to explore the world around us for tens of thousands of years, and so it is what we are used to. Second, the methods of recording visible light have been developed for nearly 200 years, whereas we still have trouble building instruments that can detect different wavelenghts like infrared and most of the more energetic (UV, x, and gamma).
A branch off of the second reason is that Earth's atmosphere is fairly transparent at visible light wavelenegths, whereas there are only some "windows" in infrared through which it is transparent, and it is almost completely opaque to UV, x, and gamma radiation (fortunately for us). Consequently, those observations need to be made in space, above our atmosphere, and so they could only be built after the dawn of spaceflight in the late 1950s.
Infrared Light
a
Low-Energy Light: Micro and Radio Waves
a
Black-Body Spectrum
a